Best solution to
identify precedent
understand approvals
de-risk strategy
We provide industry leading data and analytics to biotech and
pharmaceutical teams that are bringing oncology products to market
Trusted by well
respected companies
Data and analytics
foryour unique needs
RxTROSPECT Analytics
Ready to explore every drug approval in oncology? Our globally recognized platform empowers your team to rapidly answer questions they receive about drug approvals. It enables staff to respond faster than ever before to high-priority queries from leadership, while seeing the landscape of all drug approvals across the EU and US. This is our flagship product, a reliable login for your “need it now” calls.
Intelligence Platform
download brochure & see demo
RxTROSPECT Discovery
Ready to ingest over 25 years of data on all drug approvals in oncology? By democratizing RxTROSPECT data, our users can get access to our backend data and pull together different datasets into one place, which allows teams to discover valuable knowledge about a disease. It has been of significant value for our customers who build internal centralized knowledge platforms to consolidate diverse datasets.
Data Integration
download brochure & see demo
Choose a solution that gets you
We understand
your role & needs
Our solutions are built with your job in-mind. If you’re working on navigating regulatory strategy or exploring new opportunities in a specific therapeutic area, we know the roles you perform and the short-notice questions you get asked. We reveal the answers you need, on-demand whenever you need them.
We meet you
where you are at
Whether you’re a pre-revenue small biotech or a top 10 pharmaceutical firm, we provide access that fits your unique needs. Our existing customers include both local and global teams, who rely on our solutions for a wide array of business demands and special use cases.
zero drama,
big performance
We believe you don’t need million dollar contracts for industry leading solutions. We work with some of the most notable industry leaders, who value our turnkey tech solutions, for empowering their team to save time, cut costs, and reduce risk with data-driven decisions.
What people are saying
Testimonials are specific to an individual customer’s experience and may not be representative of all customers. Unless otherwise indicated, customers offering a testimonial do not receive compensation and their statement does not present a conflict of interest.
We are at the cutting-edge,
connect with us and get updated
Media
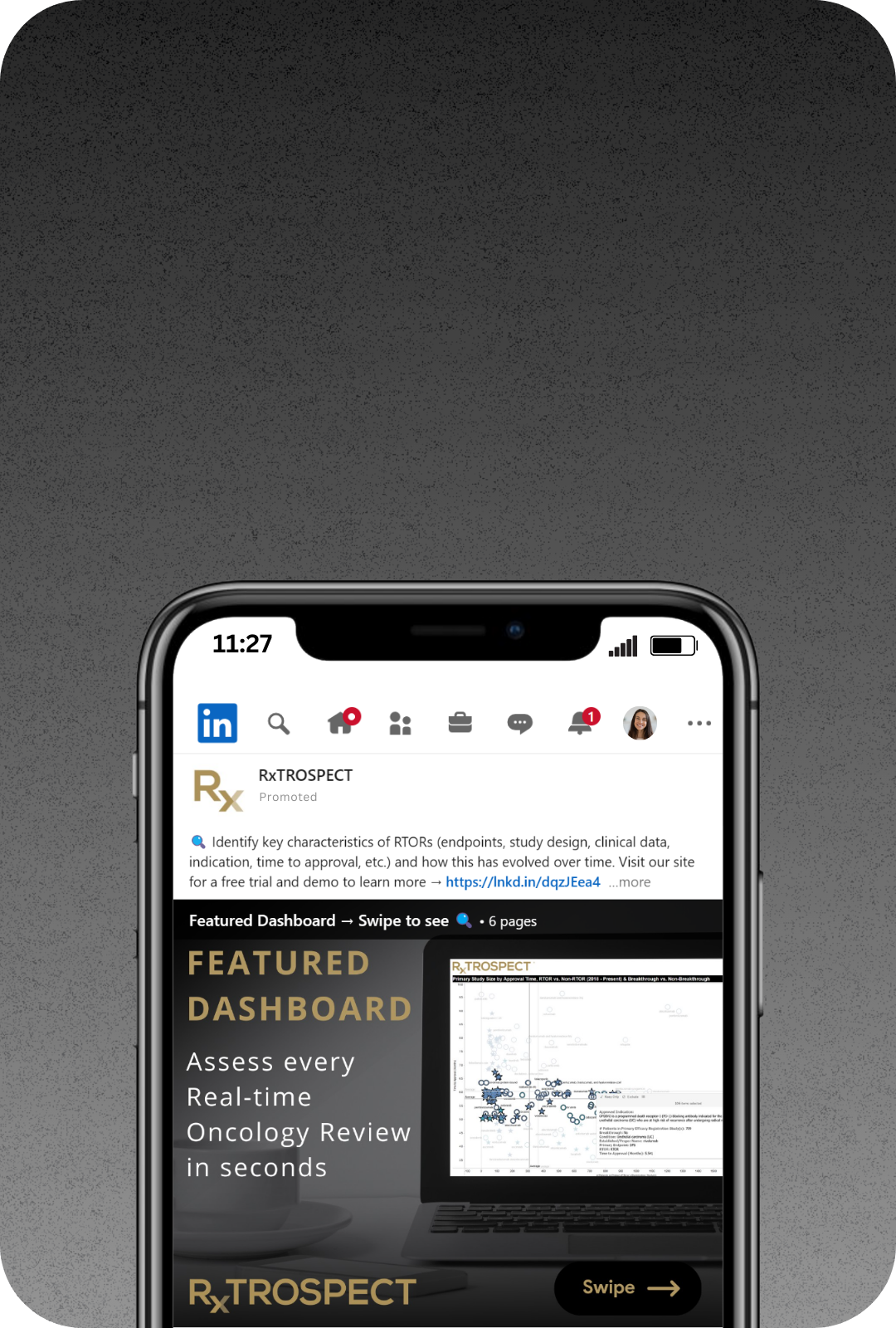
Follow us on LinkedIn
As much as we like technology, we always prefer to engage with real people. Get an invite from one of us at RxTROSPECT, we’d love to connect with you!
Get notified of key updates and new features
Join hundreds of others who get insights on what’s happening across the United States and European landscapes, with details that cover recent approvals in oncology, and much more.